What Is the Hybridization of the Central Atom in Xeo3
Why is XeO4 tetrahedral. The correct order of bond angles is.

What Is The Hybridization Of Xeo3 Quora
Sp hybridization is also called diagonal hybridization.
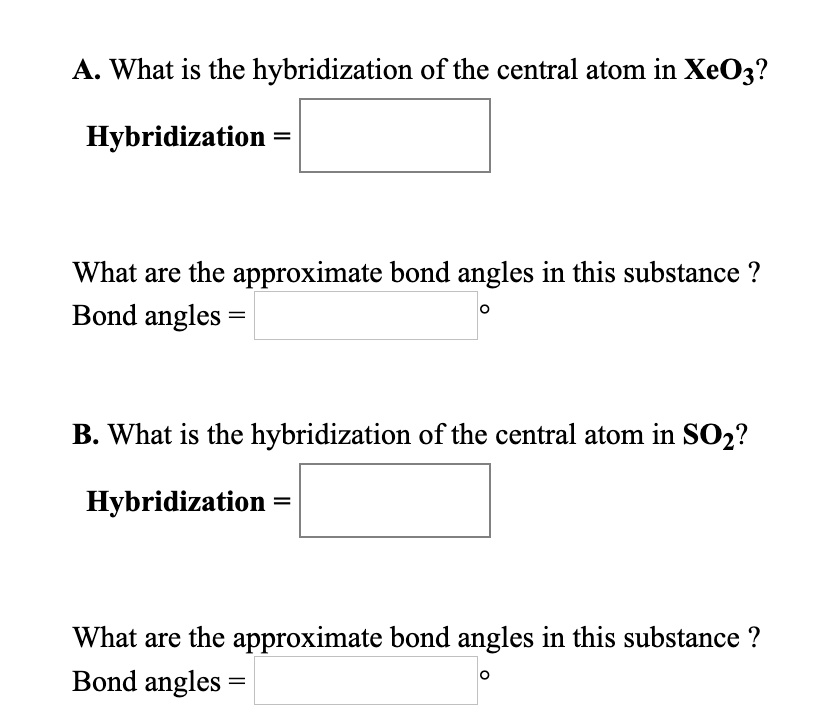
. What is the hybridization of XeO3. Predict the shape and the asked angle 9 0 or more or less in each of the following cases. This molecule is tetrahedral.
In XeO3 Xe is central atom and has 8 valence electrons. Xenon undergoes SP3 hybridization in the fourth excited in the formation of XeO4. Therefore this molecule is polar.
XeO3 is a polar molecule. NO3- too is trigonal planar. The orbitals thus formed are known as hybrid orbitals.
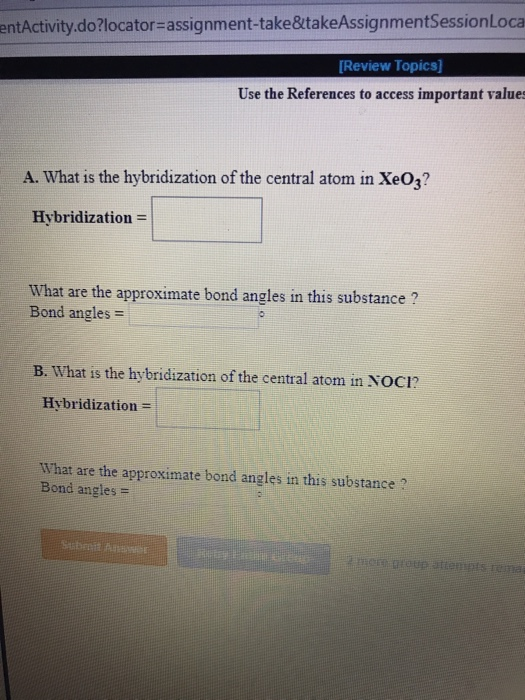
The hybridization of the central atom in NOCl is sp2. First draw the Lewis dot structure. Hybridization- What are the approximate bond angles.
The molecular geometry of XeO 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. XeO3 shows sp3 hybridization since there are 3 sigma bond pairs and 1 lone pair of electrons in the central Xe atom. MethaneThe methane molecule has four equal bonds.
S O 3 2 and the angle O S O. Hybridization- What are the. This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as an sp hybridized orbital.
In the fourth excited xenon atom has 8 unpaired electrons. The hybridization of central atom ie. What is kseo3 hybridization.
XeO3 - Xenon Trioxide. It is trigonal planar. What is Vsepr class for XeO3.
The steric number of Xenon central atom in the XeO3 molecule is 4 thus it forms Sp 3 hybridization. Place the following in order of decreasing X-A-X bond angle where A represents the central atom and X represents the outer atoms in each molecule. In kseo3 kse undergoes sp3 hybridization.
- Xenon belongs to the group of inert gases and it has the atomic number 54. CO3- the hybridisation is sp2 on carbon. No need of structure again.
The central kse atom in kseo3 has three gluing domains and only a couple of electrons. SCl2 CF4 CS2 B. A molecule containing a central atom with sp2 hybridization has an _____ electron geometry.
What is the hybridization of the central atom in XeO3. N3- no need of hybridisation. XeO3 SeBr2 The orbital hybridization on the carbon atoms in CO2 is.
The structure will be of type AB3 L. View the full answer. CS2 SCl2 CF4.
What is the expected hybridization of the central atom in a molecule of TiCl4. Hybridizationarounded atoms to central atom12valence e- of central atom -bonded e- SOhybridization of XeF661286 61. The central Xe atom in XeO3 has three bonding domains and one lone pair of electrons.
What is the hybridization of the central atom in NOCI. 1 lone pair of electrons on nitrogen 1 -bond wit. Sp2120 is the hybridization of the central atom in SO2.
Fluorine will never bond more than once so all four fluorine atoms must be bonded to the. The distribution of the charge on the central atom is asymmetric making the molecule polar. Then draw the 3D molecular structure using VSEPR rules.
CS2 CF4 SCl2 A. Hybridisation of Xe in XeO 3. Based on the types of orbitals involved in mixing the hybridization can be classified as sp 3 sp 2 sp sp 3 d sp 3 d 2 sp 3 d 3.
To find out the hybridization we will use simple forumula n2VNQ. Each sp hybridized orbital has an equal amount of s and p character 50 s and 50 p character. Does XeO3 have pyramidal shape.
Nitrogen in NOBr sp2 Explanation. CF4 CS2 SCl2 D. Find out the hybridization of X e O 3 and draw the structure accordingly.
Complete step by step answer. Calculate the number of electron pairs in the given molecule X e O 3. Start by writing electronic configuration in Xenon oxide.
So XeO3 is sp3 hybridized. Formula to calculate hybridisation -. And the shape is tetrahedral.
EntActivitydolocator-assignment-taketakeAssignmentSessionLoca Review Topics Use the References to access important value A. It is a monoatomic ion. There are a total of 7 for iodine 74 for fluorine 1 for the -1 charge 36 valence electrons on the ion.
Vvalence electron of central atom. The central atom Xe. XeO3 Xenon Trioxide.
Each oxygen atom in the XeO3 lewis structure has 4 electrons that do not involve in bonding. The hybridisation of XeO3 is sp3. C l F 3 and the angle F C l F.
Hybridisation is sp2 again. Sp3 is the molecular geometry of kseo2 which is the trigonal pyramid. Hence the electron geometry is tetrahedral and molecular geometry is pyramidal.
Since it has no lone pair of electrons the shape of XeO4 is tetrahedral with the bond angle of 109 degrees. Sp3d2 -- there are six areas of electron density around the central iodine atom four fluorine atoms and two lone electron pairs. Its hybridisation is sp3d3 however its geometry is capped or distorted octahedralsquare bipyramidal one and not pentagonal bipyramidal.
Hybridization is defined as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that redistribution of energy takes place between them which results in the formation of new orbitals with similar energies and similar shapes. Give the electron geometry molecular geometry and hybridization for H2O. The molecular geometry of XeO3 is trigonal pyramidal and its electron geometry is tetrahedral.
In BF4- the hybridisation is sp3. Hybridization is defined as the concept of mixing two atomic orbitals with the same energy levels to give a degenerated new type of orbitals. CS2 CF4 SCl2 C.
What is the hybridization of the central atom in SO2. Ie61bonded e- lonpair Sohybridization7sp3d3. What is sp3 hybridization with example.
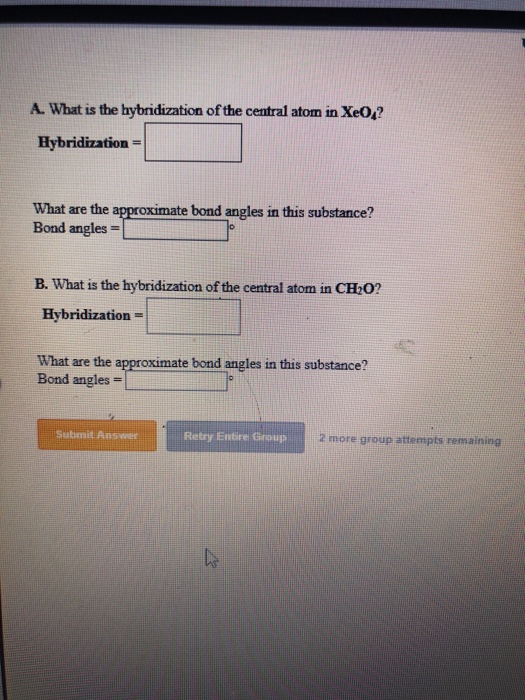
Solved A What Is The Hybridization Of The Central Atom In Chegg Com

Solved A What Is The Hybridization Of The Central Atom In Xeo3 Hybridization What Are The Approximate Bond Angles In This Substance Bond Angles B What Is The Hybridization Of The Central
No comments for "What Is the Hybridization of the Central Atom in Xeo3"
Post a Comment